Regenerative Medicine
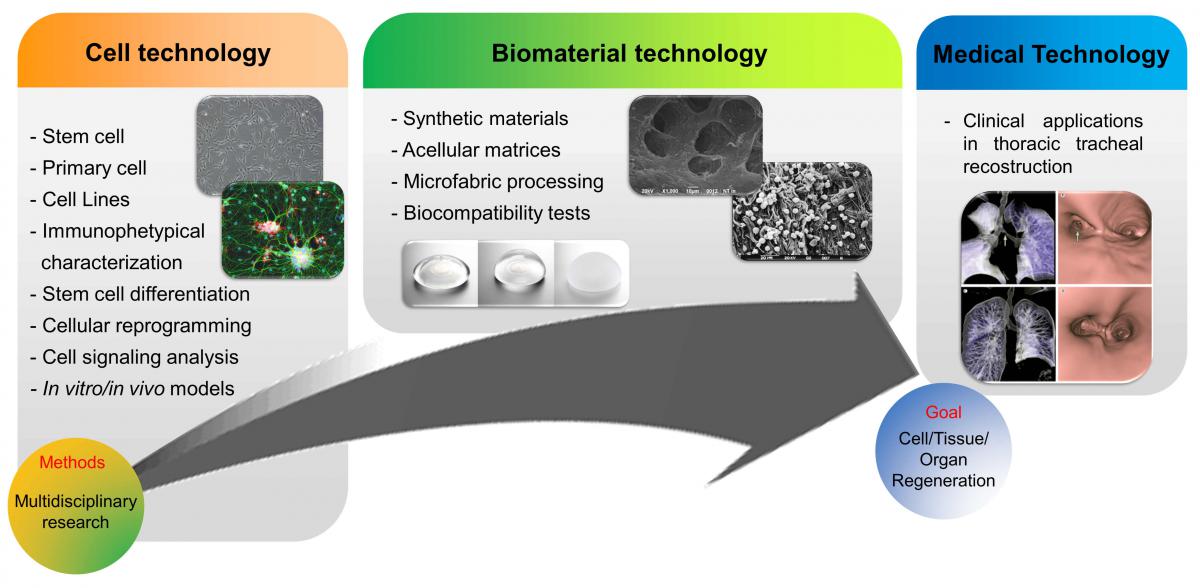
Topic: The research is mainly focused on tissue engineering to construct biological substitutes for research, diagnostic or regenerative medicine purposes and In vitro models of inflammation.
Researchers: Maria Teresa Conconi; Rosa Di Liddo
Current research interests:
- Isolation of stem cells/progenitors from human peripheral blood by High Gradient Magnetic Cell Separation (MACS) and Fluorescence activated cell sorting (FACS) for tissue engineering applications
- In vivo models for testing the regenerative potentialities of circulating multipotent stem cells
- Immunophenotyping of glial and neuronal compartments from CNS and ENS of rat and zebrafish
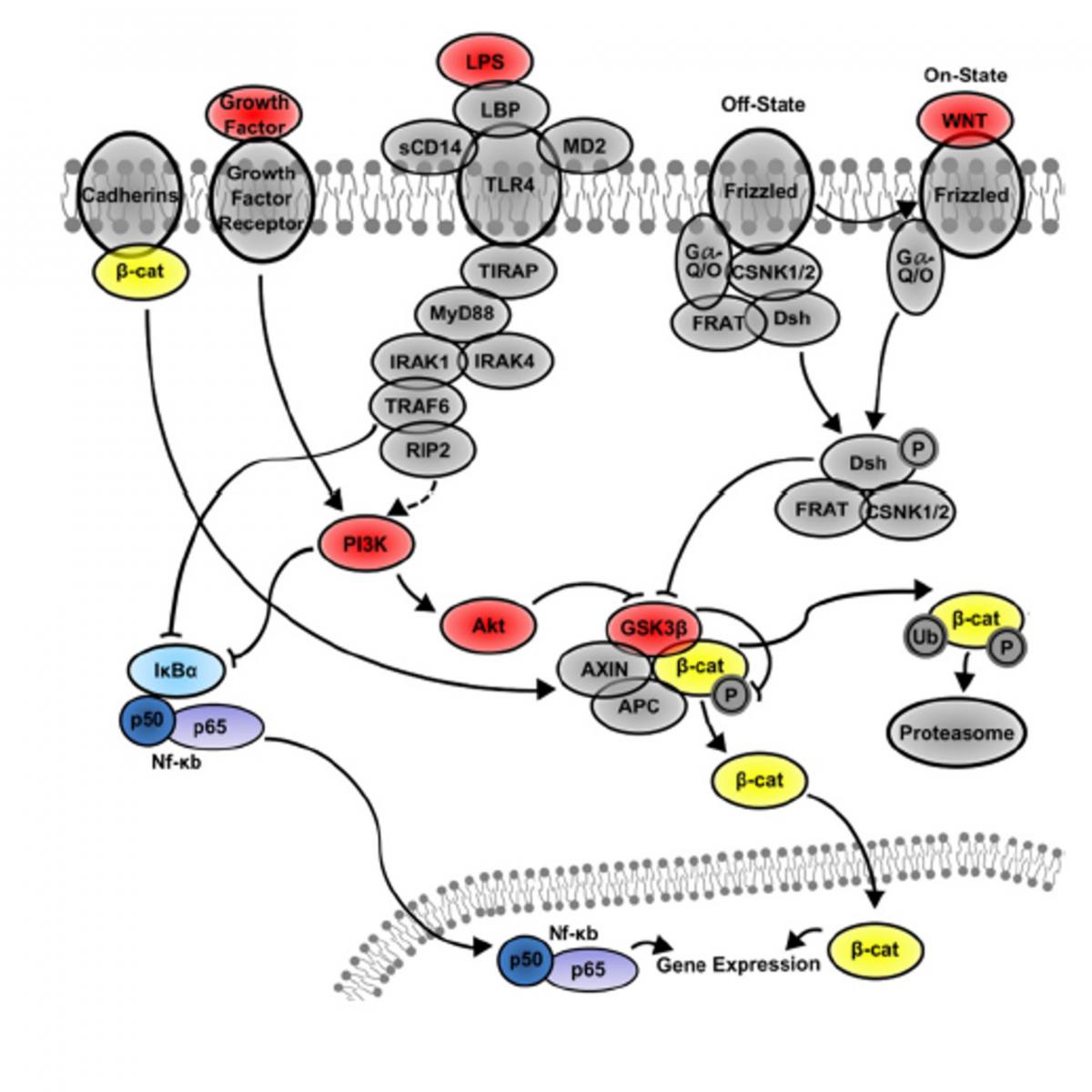
- Wnt signaling in gut inflammatory diseases
- Anti-inflammatory strategies for inhibiting the formation of silicone periprosthetic capsule
- Liver regeneration after chemical insult and biliary damage
read more
Tissue engineering (TE) is an emerging multidisciplinary field of research that combines biomedical science and engineering to construct biological substitutes for research, diagnostic or regenerative medicine purposes. A wide spectrum of TE strategies is developed in our laboratories using cells, bioactive factors and biomaterials, separately or differently combined. Primary cell cultures and adult stem cells are derived from animals (mouse, rat, pig, rabbit) or human donor tissues (bone marrow, adipose tissue, umbilical cord, peripheral blood, gut) to assemble in vitro screening systems or implant prototypes. For skeletal muscle or bone/cartilage regeneration, TE constructs have been obtained by our group combining stem cells with biomaterials providing a) proper mechanical cues (mechanical rigidity or flexibility), b) cell adhesion and migration, c) adequate porosity for diffusion of nutrients, expressed products, and waste, d) retention and presentation of biochemical factors. Synthetic biomaterials (polylactic, polyglycolic, and polycaprolactone family of polymers) have been obtained with defined physical and chemical structures through a) an optimized micro and/or nanofabrication process and b) the incorporation of soluble and bioactive factors (growth factors, bone morphogenetic proteins/BMPs). Among natural scaffolds including protein-based materials (e.g., collagen, fibrin), polysaccharide-based materials (e.g., chitosan, alginate, glycosaminoglycans, hyaluronic acid), decellularized tissue matrices showed to be a successful approach to generating a tissue-engineered organ transplant of an engineered trachea.
read more
In the last two decades, enormous progress has been made in the characterization of soluble factors regulating gut functionality at physiological and inflammed conditions. Among signaling molecules, Wnt family proteins are reported to play a pivotal role in the development and homeostasis of gut epithelium. After the binding of Wnt ligands with Frizzled/FZD receptors and LRP 5/6 proteins, β-catenin is released from the large destruction “scaffolding” complex consisting of Axin, adenomatous polyposis coli (APC), casein kinase-1 (CK1), glycogen synthase kinase-3β (GSK-3β) complex and shuttles to the nucleus promoting the transcription of target genes. Under inflammatory conditions, -catenin is shown to i) downregulate intestinal NF-кB activity, ii) reduce TNF release and iii) induce the expression of IL10 and TGF . Recently, using in vitro models of gut inflammation, our group demonstrated a neuronal surveillance through FZD9 and Wnt3a in enteric nervous system (ENS). Derived from neuronal and glial progenitors/precursors of newborn rat and mouse gut, our in vitro model could be optimized by including the epithelial and dendritic intestine components to develop versatile 3D systems for a) exploring signaling pathways active in inflammatory bowel diseases, and b) testing pharmaceutical and nutraceutical products.