lOR Institute of Oncology Research
The lOR Institute of Oncology Research is part of the Research Division of the Oncology Institute of Southern Switzerland (IOSI), the comprehensive cancer center of the Ente Ospedaliero Cantonale (EOC), and is the main facility for basic and translational research at the IOSI. The Institute is also an integral component of the clinical research activities of the IOSI participating in translational studies and multi-center clinical trials and providing expertise, facilities and technical support for pharmacodynamics, pharmacogenomics and molecular follow-up studies.

Immunology research
| The immune-research program of the Alimonti lab seeks to identify novel treatment modalities based on the dual-enhancement of senescence and immune response in prostate cancer. We have recently demonstrated that tumor-infiltrating GR1+ myeloid cells can antagonize chemotherapy-induced senescence in a model of prostate cancer (Di Mitri et al. Nature 2014). These findings have paved the way for the development of treatments that combine different immunotherapies with pro-senescence compounds and novel trials are ongoing to validate the relevance of these findings in castration resistance prostate cancer patients. In the longer run, our research will able to improve the clinical management of patients who suffer from prostate cancer. |
Immune-research group:
 |  |  | |
| Diletta Di Mitri | Arianna Calcinotto | Elena Zagato | Jinjang Chou |
Molecular Oncology Group
| Mammalian translatomes analysis on a genome-wide scale to identify key molecules involved in prostate cancer development The aim of the project is the characterization of the translatome in preclinical model of prostate cancer characterized by different cancer features and aggressiveness. Two of the most common genetic alterations in prostate cancer involve Pten and Trp53 genes: one Pten allele is deleted in up to 70% of primary prostate tumours. p53 is found completely lost or mutated almost exclusively in advanced prostate cancer. In the lab, both the Ptenpc-/- and Ptenpc-/-; Trp53pc-/- prostate cancer model have been implemented and well characterized. One of the main features of Ptenpc-/- tumor is the presence of 20/30% of cells that undergo senescence. In the Ptenpc-/-; Trp53pc-/- mouse model, cancer cells escape senescence due to Trp53 loss and progress rapidly to invasive prostate cancer with a mean survival of 5 months. We are currently studying the set of mRNAs differentially translated in Ptenpc-/- and Ptenpc-/-; Trp53pc-/- prostate cancer, to identify, first, transmembrane proteins that can be used as markers of senescence for diagnostic and therapeutic purposes. Second, tumor microenvironment has been demonstrated also in our lab to play a major role in prostate cancer development; thus, we want to further study in details the secreted factors produced by tumor cells that can play a role in leukocytes recruitment and skewing. For this purpose, we are matching polysome profiling tecnique with mass spectrometry. Polysome profiling is a well-established method of ribosome fractionation using sucrose density gradient centrifugation, by which the actively translated, ribosome-bound mRNAs can be separated by the total RNA pool. This technology, coupled with RNA sequencing analysis, allows the identification of the “translatome” of the tumors, meaning the entire pool of the ribosome-bound RNAs actively translating in the cell. By using this tecnique, it is possible to sequence more than 19000 coding and non coding RNA and, importantly, to characterize at the same time transcriptional and translational changes occurring in Ptenpc-/- and Ptenpc-/-; Trp53pc-/- prostate cancers. Preliminary results demonstrate that Ptenpc-/- and Ptenpc-/-; Trp53pc-/- tumor cells translate a different set of transmembrane and secreted proteins. Further studies will address, respectively, the role of these factors in senescence and the role in the recruitment, function and skewing of tumor-infiltrating immune cells. Concomitantly, we are applying the same approach to other preclinical prostate cancer models with different genetic background and cancer cells features generated by our lab during these years. Our final goal is to identify key molecules that can be targeted to treat prostate cancer. |
Cell Signalling group:
 | |||
| Abdullah Alajati | Daniela Brina | Jingjing Chen | Ajinkya Revandkar |
 |  | ||
| Mariantonietta D'Ambrosio | Manuel Colucci |
Pro-senescence and Senolytic Screening
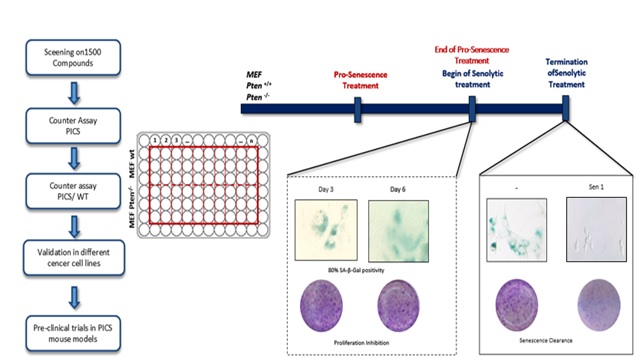 | Our laboratory mainly focusses on characterizing and subsequently manipulating pathways involved in induction of senescence, as a novel therapeutic approach in treatment of PTEN-deficient prostate tumors. Prostate cancer (PCa) at advanced stage is extremely irresponsive to conventional and targeted therapies, where chemotherapy remains of palliative benefit. These tumors often harbor PTEN-loss, which results in poor prognosis and therapy-resistance. While acute loss of PTEN induces senescence response both in vitro and in vivo, termed Pten-loss induced cellular senescence (PICS), we aim to identify novel enhancers and their target genes involved in modulating PICS. Importantly, even though senescence is demonstrated to restrict tumorigenesis in vivo, prolonged accumulation of such senescent tumor cells, have been reported to have a negative impact on the tumor microenvironment thereby allowing its progression. Thus, we aim to identify small molecule inhibitors that can selectively eliminate senescent-tumor cells. Our final aim is to implement, in tandem, a ‘Pro-senescence approach’ followed by ‘Senolytic therapy’. |
Pro-senescence and Senolytic Screening group:
 |  | ||
| Manuel Colucci | Ajinkya Revandkar | Jingjing Chen |





