Gastrointestinal Pharmacology
Topic: Understanding of mechanisms regulating gastrointestinal function in health and disease to develop new pharmacological treatments or nutritional strategies for disorders of the gut-brain axis, obesity, inflammatory bowel disease and neurodegenerative diseases.
Researchers: Rocchina Colucci - Cecilia Giron
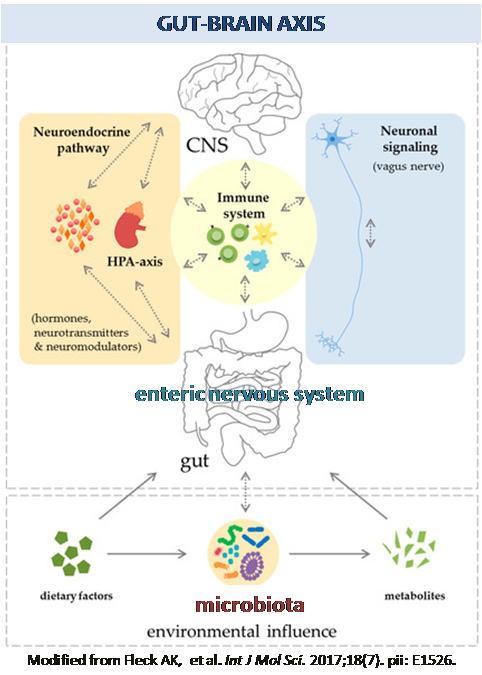
The overall focus of our research is to study the mechanisms regulating gastrointestinal function in health and disease in order to develop new pharmacological treatments or nutritional strategies. The main areas of current research apply to gut-brain axis-related disorders (i.e. irritable bowel syndrome), obesity, inflammatory bowel diseases (i.e. Crohn’s diseases and ulcerative colitis) and neurodegenerative disease (e.g. Parkinson’s disease and Alzheimer’s disease). We employ a variety of experimental methodologies at the cellular, whole tissue and in vivo level. The techniques employed comprise electrophysiology, molecular biology, immunohistochemistry, organ bath, and in vivo gastrointestinal motility.
| Current research interests: | |
|  |
 | |
 | Neurodegenerative disease (Parkinson’s disease and Alzheimer’s disease) |
read more
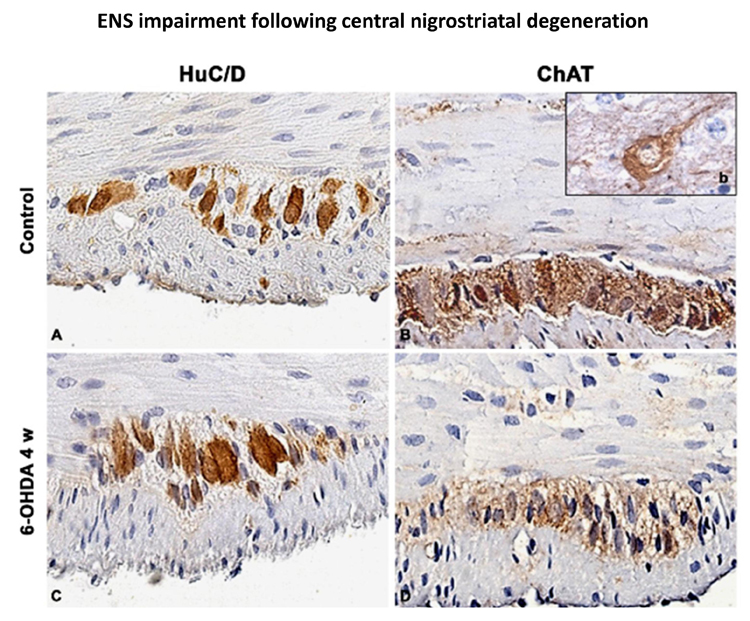
Parkinson’s disease (PD) is a common neurodegenerative disorder, characterized by tremor, bradykinesia, and rigidity. PD is also associated with gastrointestinal (GI) dysmotility, including dysphagia, constipation, and defecatory disorder, which contribute to PD morbidity (Braak et al., 2006; Cloud and Greene, 2011; Pellegrini et al., 2015). This project embraces a multidisciplinary approach including biochemical and neuromolecular studies in models of neurodegenerative diseases in collaboration with Prof. Blandizzi (Pharmacology, University of Pisa); Prof. Bernardini (Histology, University of Pisa), Prof. Scarpignato (Pharmacology, University of Parma).
Colucci
Selected publications:
Pellegrini C et al. Effects of L DOPA/benserazide co-treatment on colonic excitatory cholinergic motility and enteric inflammation following dopaminergic nigrostriatal neurodegeneration. Neuropharmacology. 2017. 123: 22-33.
Pellegrini C et al. Alteration of colonic excitatory tachykininergic motility and enteric inflammation following dopaminergic nigrostriatal neurodegeneration. J Neuroinflammation. 2016;13 :146.
Fornai M et al. Enteric Dysfunctions in Experimental Parkinson's Disease: Alterations of Excitatory Cholinergic Neurotransmission Regulating Colonic Motility in Rats. J Pharmacol Exp Ther. 2016; 356: 434-44.
 | |
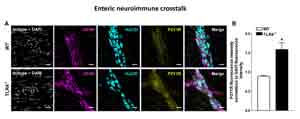 | Neuroimmune crosstalk in inflammatory bowel diseases |
read more
Inflammatory bowel diseases (IBD) are recurring debilitating inflammatory conditions. IBD etiology is still vague and involves the complex interaction of genetic, environmental, immunoregulatory and microbiome-derived factors. The enteric nervous system (ENS) is increasingly recognized as a key regulator of immune responses. Indeed, ENS through the production/release of several neurotransmitters, including dopamine and serotonin, can modulate the immune system and be a major contributor to IBD pathogenesis. The majority of IBD-associated specific mutations includes genes involved in microbial recognition, such as mutations in the Toll-like receptors (TLRs). Beside controlling host-defense responses, TLR4 modulates ENS activity, gut motility and repair processes following an insult. We have recently discovered that TLRs deficiency in mice leads to significant structural and functional ENS alterations, characterized by modified gut motility and susceptibility to inflammation. Therefore, we intend to investigate whether a TLRs-mediated ENS dysfunction is responsible in affecting IBD pathogenesis. This project embraces a multidisciplinary approach including biochemical and neuromolecular studies in models of colitis and IBD patients to unravel the gut neuroimmune signature of IBD, in collaboration with Prof. Savarino and Prof. D'Incà (Gastroenterology, University of Padova), Prof. Rugge (Pathology, University of Padova).
Colucci, Giron
Selected publications:
Caputi V et al. Antibiotic-induced dysbiosis of the microbiota impairs gut neuromuscular function in juvenile mice. Br J Pharmacol. 2017;174(20):3623-3639.
Caputi V et al.Toll-Like Receptor 4 Modulates Small Intestine Neuromuscular Function through Nitrergic and Purinergic Pathways. Front Pharmacol. 2017; 8:350.
Brun P et al. Toll-like receptor 2 regulates intestinal inflammation by controlling integrity of the enteric nervous system. Gastroenterology. 2013; 145 :1323-33.
read more
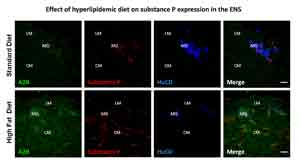
Obesity is an epidemic challenge for global public health, triggering higher morbidity and mortality with consequent reduction of life expectancy. The mechanisms by which diabetes causes neuronal injury are, however, poorly understood. Although the mechanisms are unclear, emerging evidence show the host microbioma as one of the main actor involved in modulating gut homeostasis, metabolic status and central nervous system fitness. Until now the possible relationship between maternal obesity followed by a high-fat diet during childhood and the predisposition to develop brain and gut disturbances in adulthood has been scarcely investigated. Our preliminary data show that animals receiving a high-fat diet have reduced neuromuscular contractility, slower intestinal transit and gut neuropathy that parallel the changes in gut microbioma. This project embraces a multidisciplinary approach including biochemical and neuromolecular studies in models of obesity in collaboration with Prof. Blandizzi (Pharmacology, University of Pisa); Prof. Bernardini (Histology, University of Pisa), Prof. Scarpignato (Pharmacology, University of Parma).
Colucci, Giron
Selected publications:
Antonioli L. et al. Colonic motor dysfunctions in a mouse model of high-fat diet-induced obesity: an involvement of A(2B) adenosine receptors. Purinergic Signal. 2017. Aug 14. doi: 10.1007/s11302-017-9577-0.
Antonioli L. et al. Colonic dysmotility associated with high fat diet-induced obesity: role of the enteric glia. Gastroenterology 2017; 152 (5, Suppl 1): S180.
Antonioli L. et al. Alterations of colonic neuromuscular excitatory tachykininergic pathways in a mouse model of diet induced-obesity. Gastroenterology 2016; 150 (4, Suppl 1): S351.
read more
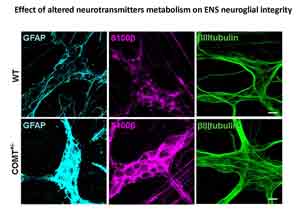
The gut microbiota of the infant, acquired by exposure to the mother and the early rearing environment, plays a critical role in establishing a functional gastrointestinal tract and promoting health by stimulating the maturation of the immune, endocrine, enteric and central nervous system. We hypothesize that incorrect microbial colonization of the gut may exacerbate or drive behavioral deficits by promoting aberrant neuronal circuitry and function via the gut-brain axis during neonatal and pediatric age. Thus, changes in the composition of the maternal microbiome may play a role in the development of mental disorders during childhood, such as the attention-deficit/hyperactivity disorder (ADHD). On the other hand, disturbances affecting central nervous system (e.g. changes in catechol-O-methyl transferase activity) may be involved in the pathogenesis of functional bowel disorders. The main goal of this research area is to understand the effectiveness and feasibility of mental health improvement as a function of microbial modulation. This project embraces a multidisciplinary collaboration with Dr. Cristina Giaroni (University of Insubria, Varese) and Dr. Francesco Papaleo (Institute of Technology, ITT, Genova) and, involves an array of techniques ranging from neuroscience and neurogastroenterology to microbiology and food and nutrition sciences.
Giron
Selected publications:
Caputi V et al. Dopamine transporter genetic reduction affects small-bowel neuromuscular contractility in mice. Neurogastroenterology and Motility 2017, 29 (Supplement 2): 17.
Caputi V et al. Gut dysmotility after catechol-O-methyltransferase and dopamine transporter genetic reduction in mice: implication in irritable bowel syndrome pathogenesis. United European Gastroenterology Journal 2015; 3(5S): A639.
Caputi V et al. Involvement of catechol-O-methyltransferase genetic reduction in murine intestinal dysmotility: a possible link between psychiatric disorders and irritable bowel syndrome. Gastroenterology 2015; 148 (4, Suppl 1): S774-S775.