Cancer Pharmacology
Topic: Pharmacodynamics and pharmacokinetic aspects of cancer pharmacology, with special emphasis on discovery of novel molecular targets in cancer cells and in the non-cancerous cells forming the tumor microenvironment, cancer metabolomics, metabolite profiling and evaluation of drug-drug interaction potential of anticancer agents.
Researchers: Monica Montopoli; Lucia Trevisi, Luigi Quintieri
Current research interests:
- Selective induction of controlled cancer cell death
- Pharmacologic modulation of angiogenesis
- Cancer metabolism and drug resistance
- Drugs targeting the tumor microenvironment
- Cancer metabolomics
- Drug metabolite profiling and assessment of potential metabolism-based drug-drug interactions
 |  |
Selective induction of controlled cancer cell death Cancer cells are highly adaptable and activation of survival signaling pathways or inactivation of death signaling pathways can lead to resistance to chemotherapeutics. The aim of the research is to identify novel molecular targets as well as new anticancer drugs, affecting survival and death pathways in cancer cells, that can potentially overcome intrinsic and acquired chemoresistance. |
read more
The JNK kinases are known to control several cellular processes including apoptotic cell death. Among the endogenous modulators of JNK activity there is the cytosolic glutathione transferase named GSTP1-1, aprotein overexpressed in several human tumors, and known to be capable of sequestering and inactivating JNK. Interestingly, some GSTP1-1-interacting agents have been found to be potent apoptosis inducers in cancer cells, and highly effective and safe antitumor agents in animal models. Current research activity in this area include the in vitro screening of selective human GSTP1-1 inhibitors for cancer therapy.
Luigi Quintieri
Increasing evidence suggest that Na+/K+-ATPase (Na pump) of cancer cells could be a target for anticancer therapy. Besides its pumping function, Na+/K+-ATPase acts as a signal transducer, controlling cell survival, proliferation and differentiation. In cancer cells, cardiac glycosides (specific ligands of Na+/K+-ATPasi) initiate multiple signaling cascades that induce cell proliferation arrest and cell death by apoptosis, anoikis or autophagy. The postulated mechanisms are unrelated to Na pump inhibition and include: activation of the kinases Src, JNK, p38 MAPK and the Ras-Raf-MEK-ERK cascade; production of mitochondrial reactive oxygen species (ROS); decrease of the antiapoptotic protein Bcl-2; increase of caspase activation. Our research in this area covers: in vitro testing of clinically used cardiac glycosides (digoxin, digitoxin, ouabain) on different human cancer cell lines and study of the molecular pathways involved in cancer cell death; elucidation of the role of altered Na+/K+-ATPase expression, in particular of the catalytic α-subunit (target of cardiac glycosides), in neoplastic transformation and cardiac glycoside sensitivity.
Lucia Trevisi
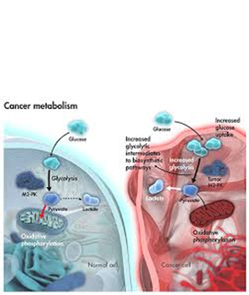 |  |
Cancer metabolism and drug resistance Cancer metabolism refers to the alterations in cellular metabolism pathways that are evident in cancer cells compared with most normal tissue cells...
|
read more
Cancer metabolism refers to the alterations in cellular metabolism pathways that are evident in cancer cells compared with most normal tissue cells. Metabolic alterations in cancer cells are numerous and include aerobic glycolysis, reduced oxidative phosphorylation and the increased generation of biosynthetic intermediates needed for cell growth and proliferation. In the recent years, a growing body of experimental and clinical evidence has been obtained suggesting that the metabolic plasticity of malignant cells plays a key role in cancer progression and in drug resistance. This metabolic plasticity is central to different networks, not only those directly pertaining to metabolism (including autophagy), but also others referring to broader aspects of cancer cell biology, as the metabolic activities of cancer cells directly influences genetic, epigenetic, intra- and inter-cellular signaling and the response of tumors to different anticancer therapies. Novel therapeutic targets have been identified and new treatments are being developed and/or validated, bringing new hope to selectively target different metabolic determinants that control phenotypes associated to tumor progression and drug resistance.
 |  |
Drugs targeting the tumor microenvironment For many years cancer pharmacology was mainly devoted to the study of neoplastic transformation and drugs targeting tumor cells. Nowadays it is recognized that stromal (non-cancerous) cells forming the tumor microenvironment, such as tumor-associated macrophages, endothelial cells, pericytes, fibroblasts and immune cells, play a fundamental role in cancer progression and development. |
read more
In fact, tumor cells recruit stromal cells by secreting chemokines and growth factors, which stimulate them to create a tumor-favoring microenvironment. The interplay among different stromal and cancer cells contributes to tumor angiogenesis, tumor growth, metastasis and development of resistance to chemotherapy. Targeting stromal cells, especially the mechanisms involved in the generation of a pro-tumoral microenvironment, represents a valuable strategy for cancer treatment.
The aim of the research is to study drugs affecting the tumor microenvironment acting on endothelial cells, by modulation of pro-angiogenic signaling pathways, or inflammatory cells, by reprogramming macrophages to anti-tumoral phenotypes. The research is conducted using human primary cells (HUVEC, monocytes, macrophages) and different cancer cell lines. The effect of drugs on the interplay between different types of stromal cells and between stromal and cancer cells is studied using co-culture systems. Furthermore, analysis of the secretome composition and biological activity is performed to investigate the molecular mediators involved in cellular interactions.
Lucia Trevisi in collaboration with Chiara Bolego
 | |
 | |
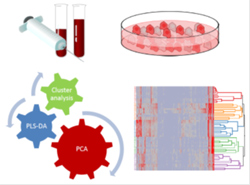 | Cancer metabolomics The metabolome is the set of all metabolites of an organism, such as gene expression result. While gene expression data and proteomic determinations are notalways able to fully explain the result of cellular activities, conversely metabolomic profile can provide a snapshot of the integrated cell functions...
|
read more
The metabolome is the set of all metabolites of an organism, such as gene expression result. While gene expression data and proteomic determinations are notalways able to fully explain the result of cellular activities, conversely metabolomic profile can provide a snapshot of the integrated cell functions. By studying metabolites that cellular proteins produce, and which are differently expressed in disease states, a targeted strategy can be outlined, with the goal of a personalized pharmacotherapeutic intervention. The aim of the research is therefore the determination of the metabolomic profile characteristic of the diseases, such as cancer and dysmetabolic pathologies, to obtain the characterization of specific markers, and subsequently, through them, to evaluate the effectiveness of therapeutic interventions to cure and hope fully prevent the disease. The method consists of analytical measurements conducted on tissue or biological fluid samples obtained from patients, but also from specificcancercellcultures, followed by multivariate techniques of data analysis (data mining).
Eugenio Ragazzi, Monica Montopoli
 |  |
Drug metabolite profiling and assessment of potential metabolism-based drug-drug interactions Biotransformation is a major determinant in both pharmacokinetics and therapeutic and toxic response to the majority of drugs. |
read more
Knowledge about the metabolism of a new chemical entity and its affinity to drug-metabolizing enzymes is valuable in the drug discovery and development processes by providing essential information for the selection of candidates from among a number of compounds similarly effective in their therapeutic potential. In particular, in vitro metabolism studies help in extrapolating the in vivo animal pharmacokinetics to humans, and provide an insight into possible interspecies differences in drug toxicity and therapeutic efficacy. Furthermore, identification of enzymes involved in the major metabolic pathways of a candidate, and execution of in vitro metabolism-based drug-drug interaction studies may help in predicting the probable drug-drug interactions in humans. Current research activity in this area include the early characterization of the metabolic fate of novel GSTP1-1 and tubulin polymerization inhibitors for cancer therapy, to support medicinal chemistry efforts in designing more metabolically-stable candidates.
Luigi Quintieri





