Galenic and Cosmetic Formulation and Pharmaceutical Processes
Topic: The main focus is the design and development of formulations, dosage forms as well as the set-up of technological and industrial processes for production of pharmaceutics.
Researchers: Erica Franceschinis; Alessandra Semenzato
Current research interests:
- Mechanisms underlying the wet granulation process in order to develop new scale-up strategies
- Novel oral solid dosage forms (lipidic formulations and solid dispersions)
- Strategies to improve the dispersion of high hydrophilic molecules (hydrocolloids) in food and pharmaceutical industrial processes
- topical systems for cosmetic and pharmaceutical applications
- Rheological studies on new cosmetic ingredients or finished products
- Stability, usability and efficacy testing
- Preparation and characterization of delivery systems for topical application
- Procedures for industrial and extemporaneous radiopharmaceuticals production
- Pharmaceutical legislation
 |  |
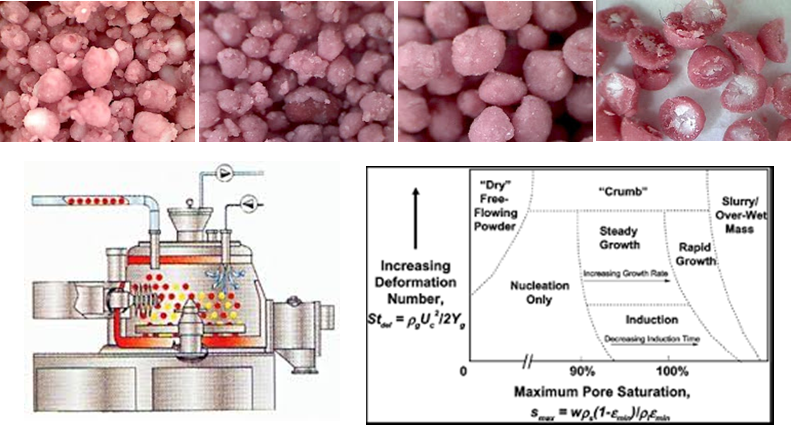 | Wet Granulation and scale up Granulation is defined as ‘‘any process whereby small particles are gathered into larger, permanent masses in which the original particles can still be identified.” During the granulation fine powders are agglomerated into larger granules to produce engineered particles with superior attributes (e.g. improved appearance, flow properties, mixedness). This process represents an example of particle design where the desired attributes of the granule are controlled by a combination of the formulation and the process. |
read more
Among the different granulation techniques, the wet granulation in high shear mixers is one of the most employed in pharmaceutical industries to increase the uniformity of drug distribution, the material density and compressibility, as well as to enhance the flowability and to reduce dust. This is due to the fact that high shear mixers present some advantages if compared to other techniques such as the possibility to work with wet and sticky materials and with viscous binders but moreover to produce small and high-density granules. Over the past decade there have been substantial gains in understanding of the granulation process, however design, scale-up and granulation processes still need efforts before being considered as fully quantitative engineering. Scale-up process is the basis of the expansion of production capacities and hence of the commercialization of new products. Scale-up of any pharmaceutical process is a great technical and economic challenge and the current approach based on dimensionless numbers is not effective. Consequently the development of new strategies, e.g. the creation of new dimensionless numbers based on material properties not yet considered and easy to calculate, could represent a challenge (Erica Franceschinis).
 |  |
 | Development of lipid formulations Among lipid formulations, self-emulsifying systems (SEDDS) are the most promising because in presence of gastro-intestinal fluids they potentially form an oil-in-water microemulsion increasing the active molecule dissolution and permeation |
read more
The development of SEDDS involve initially the selection of suitable excipients such as oil, surfactants, co-surfactants and co-solvent able to dissolve a large amount of active molecule and to emulsify spontaneously in the presence of water. Afterward phase diagrams are generally constructed in order to identify the mixtures able to emulsify and then a detailed characterization (e.g. drop size and ξ potential, emulsification rate, stability, dissolution rate,..) of the obtained system is necessary to select the best formulation. To overcome the disadvantage of these formulations (e.g. high manufacturing costs, stability problems) SEDDS can be absorbed into powders in order to produce solid-SEDDS by different techniques (Erica Franceschinis)
 |  |
 | Design and development of oral solid dosage forms |
read more
Nevertheless during the development of an oral dosage form it is important to take into account different parameters related to the active molecule properties, such as solubility and permeability, but also its therapeutic dosage, metabolism and stability. Consequently during the development of an oral solid dosage form an extensive active molecule characterization is necessary (solubility, permeability, crystalline state, flowability, compressibility, …) and its compatibility with different excipients must be evaluated by different techniques (DSC, XRPD). On the basis of these parameters the most appropriate solid dosage (granules, tablet, capsule) form is selected, produced and characterized evaluating e.g. the crushing strength, swelling ability, friability, disintegration time and the active molecule dissolution rate. (Erica Franceschinis, Marisa Dal Zotto).
 |  |
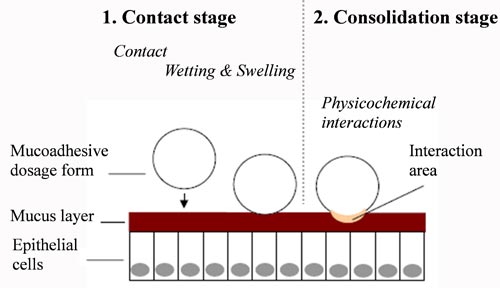 | Mucoadhesive tablets Over the last decades mucoadhesive drug delivery systems have become of interest for their potential to increase the dosage form residence time at a specific location in the body that can significantly contribute to achieve a greater and prolonged therapeutic effect, reduce the frequency of administrations and increase the effective of the local disorders treatment. |
read more
Mucoadhesion is a complex phenomenon, not yet fully understood, consisting of a combination of different interaction mechanisms. The mucoadhesive properties of mucoadhesive drug delivery systems are due to the presence of mucoadhesive substances, generally hydrophilic polymers presenting hydrophilic groups or charged groups able to interact with the mucus layer when the polymer hydration occurs. Consequently, the success and the degree of mucoadhesion is influenced by different polymer properties (e.g. molecular weight, crystalline state, swelling ability and extent) that must be evaluated during formulation studies.
Consequently, during the development of such systems it is necessary an extensive polymer characterization in order to evaluate molecular weight, crystalline state, swelling ability and extent in different medium and finally the mucoadhesive properties.
Although many methods have been developed to evaluate mucoadhesion, pharmacopoeia methods and standard apparatus are not yet available, hence the development of a robust and reproducible detection method represent a challenge. (Erica Franceschinis)
 |  |
 | Technological development of β emitting radiopharmaceutical produced with radioactive ion beams An innovative method is proposed for the production of carrier-free b- radiopharmaceuticals for therapy, based on the Isotope Separation On-Line (ISOL) technique in the framework of the SPES (Selective Production of the Exotic Species) project. SPES is a nuclear physics project under advanced construction at Legnaro National Laboratories of INFN (Istituto Nazionale di Fisica Nucleare). |
read more
The innovation of the method relies not only on the new physical technique but mainly on the final medicinal product. The class of radiopharmaceuticals obtainable, indeed, can be considered completely new, since they have a high purity in terms of specific activity, that is the ratio between radioactivity and mass of the element. Radiopharmaceuticals produced with the proposed method have specific activity values 5 orders of magnitude bigger than the traditional ones.
The involvement of the Pharmaceutical Technology group is devoted to the production of the radiopharmaceuticals taking into account the European legislation for the regulation of medicines. In particular, the production method, the constituents and containers must be in accordance with the technical standards that define the quality of medicinal products, as the basis for their therapeutic efficacy and safety (Nicola Realdon, Erica Franceschinis).
 |  |
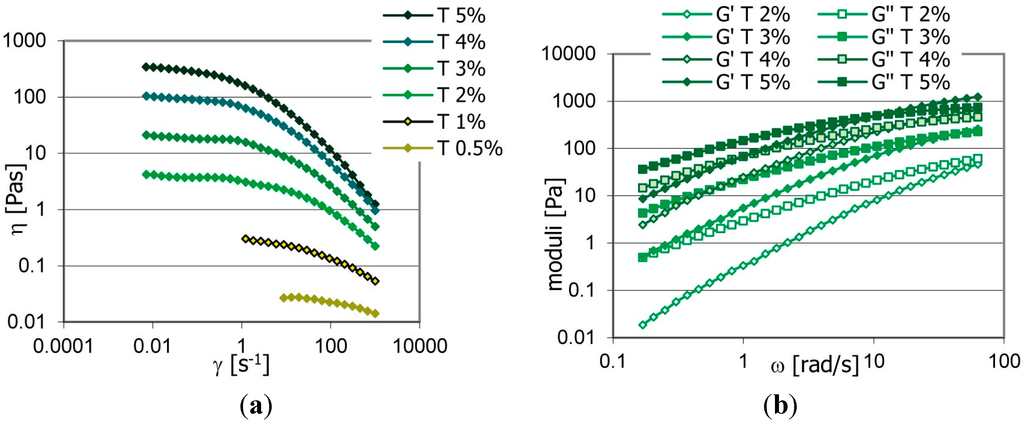
 | Cosmetic Lab Formulation development and rheological studies to improve the performance of topical formulations on skin and mucosal tissues using technological innovative solutions |
 |
|
read more
In a context of challenging business environments, unstable markets, small profits, and fierce competition, firms developing new formulas necessitate safety as well as the prospect for success.
Rheological studies applied to product development represent a powerful tool to achieve formulation improvement in terms of stability, usability and efficacy.
Cosmetic Lab provides research, consulting and technical support to R&D department of cosmetic and pharmaceutical companies for:
- New formulations development
- Optimization of the ingredients selection
- Sustaining product claims
- Comparison with benchmark products
- Evaluation of functional properties (i.e. moisturization, mucoahesion, film forming properties….)
- Evaluation of usability
- STABILITY TESTING
(Alessandra Semenzato)





